
Polish version
 Polish version |
| Previous Protein isoelectric point calculator | Index Protein isoelectric point calculator | Next Examplary program calculating protein isoelectric point |
- for negative charged macromolecules:
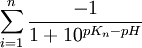
- for positive charged macromolecules:
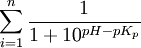
| Amino acid | NH2 | COOH | C | D | E | H | K | R | Y |
| EMBOSS | 8.6 | 3.6 | 8.5 | 3.9 | 4.1 | 6.5 | 10.8 | 12.5 | 10.1 |
| DTASelect | 8.0 | 3.1 | 8.5 | 4.4 | 4.4 | 6.5 | 10.0 | 12.0 | 10.0 |
| Solomon | 9.6 | 2.4 | 8.3 | 3.9 | 4.3 | 6.0 | 10.5 | 12.5 | 10.1 |
| Sillero | 8.2 | 3.2 | 9.0 | 4.0 | 4.5 | 6.4 | 10.4 | 12.0 | 10.0 |
| Rodwell | 8.0 | 3.1 | 8.33 | 3.68 | 4.25 | 6.0 | 11.5 | 11.5 | 10.07 |
| Patrickios | 11.2 | 4.2 | - | 4.2 | 4.2 | - | 11.2 | 11.2 | - |
| Wikipedia | 8.2 | 3.65 | 8.18 | 3.9 | 4.07 | 6.04 | 10.54 | 12.48 | 10.46 |
| Lehninger | 9.69 | 2.34 | 8.33 | 3.86 | 4.25 | 6.0 | 10.5 | 12.4 | 10.0 |
| Grimsley | 7.7 | 3.3 | 6.8 | 3.5 | 4.2 | 6.6 | 10.5 | 12.04* | 10.3 |
* Arg was not included in the study and the average pK from all other scales was taken
More advanced algorithm, implemented in ProMoST, takes into account localization of the charched amino acid:
| aa | N term | middle | C term |
| K R H D E C U* Y | 10.00 11.50 4.89 3.57 4.15 8.00 5.20 9.34 | 9.80 12.50 6.08 4.07 4.45 8.28 5.43 9.84 | 10.30 11.50 6.89 4.57 4.75 9.00 5.60 10.34 |
| * pK was taken from Byun et al. 2011 | |||
| aa | N | C | aa | N | C | aa | N | C | aa | N | C | |||
| G A S P | 7.50 7.58 6.86 8.36 | 3.70 3.75 3.61 3.61 | V T I L | 7.44 7.02 7.48 7.46 | 3.69 3.57 3.72 3.73 | N Q M F | 7.22 6.73 6.98 6.96 | 3.64 3.57 3.68 3.98 | W X* Z** B*** | 7.11 7.26 6.96 7.46 | 3.78 3.57 3.54 3.57 | |||
| * X - average from all amino acids ** Z =(E+Q)/2 *** B =(N+D)/2 | ||||||||||||||
Now, having this few peaces of information we can try to write simple computer program which calculate isoelectric point. We will use free compiler DevC++ as the program will be written in C++ programming language. To read next section you should have at least basic knowledge in C++.
For more theoretical information go to:
http://en.wikipedia.org/wiki/Isoelectric_point
Tabb DL (2001) An algorithm for isoelectric point estimation
Sillero A, Maldonado A. (2006) Isoelectric point determination of proteins and other macromolecules: oscillating method. Comput Biol Med. 36(2), 157-66. Epub 2005 Jan 1 - this one is not open access article
Grimsley GR, Scholtz JM, Pace CN. A summary of the measured pK values of the ionizable groups in folded proteins. Protein Sci. 2009 Jan;18(1):247-51.